Dimethyl Sulfide – the surprising compound behind ocean smells and more
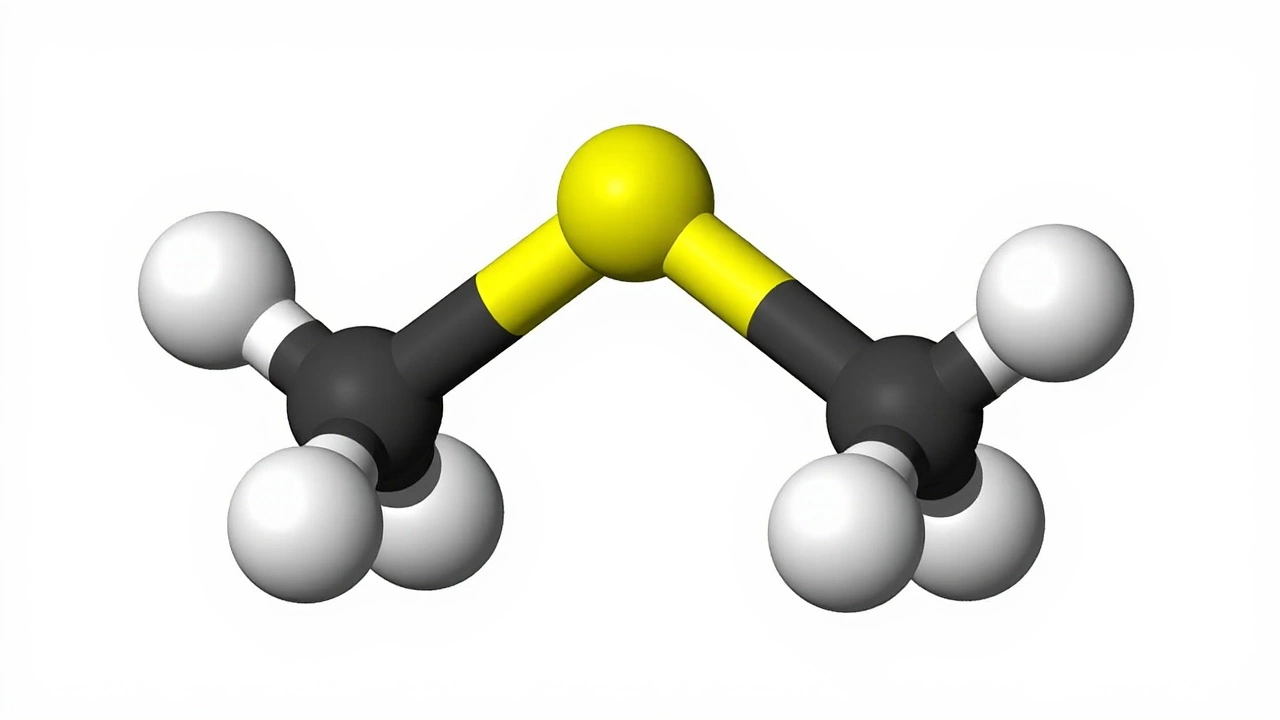
Ever wonder why the sea sometimes smells like garlic or rotten eggs? That scent comes from a tiny chemical called dimethyl sulfide, or DMS for short. It’s a simple molecule made of two carbon atoms and a sulfur atom, but it plays a big part in nature and industry. In this guide you’ll get the basics of where DMS comes from, why scientists care about it, and how it shows up in everyday life.
Where dimethyl sulfide comes from
Most DMS is produced by tiny plants called phytoplankton that float in the upper ocean. When these microorganisms grow, they create a compound called dimethylsulfoniopropionate (DMSP). As DMSP breaks down, it releases DMS into the water and then into the air.
That release isn’t just an odd smell. DMS can rise high enough to become part of cloud formation. Some researchers think this helps the Earth stay cool by reflecting sunlight back into space – a natural climate‑regulating loop. On land, DMS also forms when certain bacteria decompose organic matter in soils and wetlands.
How dimethyl sulfide is used
Beyond nature, DMS is a handy ingredient in several industries. Its distinctive odor makes it a useful tracer for detecting gas leaks in pipelines. In the food world, a tiny amount adds a tasty, buttery flavor to popcorn and certain cheeses. It’s also a building block for making other chemicals, like the solvent dimethyl sulfide oxide, which shows up in paints and inks.
If you’ve ever noticed a strong smell in a fish market, that’s DMS too. The compound breaks down from fish oils, giving that characteristic “sea‑food” aroma. In the lab, scientists use DMS to study how sulfur moves through ecosystems and to model how climate‑active gases behave.
So, why should you care? Knowing about DMS helps you understand why the ocean smells the way it does, how tiny organisms can influence global temperatures, and where that smell ends up in everyday products. Next time you walk along a beach and catch a whiff of something pungent, you’ll know it’s dimethyl sulfide doing its quiet work.
Recent findings by a research team from the University of Bern reveal the presence of dimethyl sulfide (DMS) in comet 67P/Churyumov-Gerasimenko, challenging its role as a biosignature. Utilizing data from the Rosetta mission, researchers showed that DMS can form through abiotic processes. This discovery, coupled with DMS detection in the interstellar medium, stresses the need for careful evaluation of biosignatures in space.
Continue Reading





